Abstract
Background - The WINDOW-1 regimen introduced first-line ibrutinib with rituximab (IR) followed by 4 cycles of R-HCVAD for younger mantle cell lymphoma (MCL) patients (pts) demonstrating 90% CR on IR alone and we aimed to improve the CR rate with the addition of venetoclax. We therefore investigated the efficacy and safety of IR and venetoclax (IRV) followed by risk-stratified observation or short course R-HCVAD/MTX-ARA-C as consolidation in previously untreated young patients with mantle cell lymphoma (MCL). Our aim was to use a triplet chemotherapy-free induction to reduce the toxicity, complications and minimize chemotherapy exposure in MCL pts.
Methods - We enrolled 50 previously untreated pts in this single institution, single arm, phase II clinical trial - NCT03710772. Pts received IR induction (Part-1) for initial 4 cycles. Pts were restaged at cycle 4 and received IRV for up to eight cycles (Cycle 5 to Cycle 12) starting with ramp up venetoclax dosing in Cycle 5. All pts who achieved CR prior to cycle 12 continued to receive IRV for 4 cycles (maximum 12 cycles) and then moved to part 2. Pts were stratified into three disease risk groups: high, moderate and low risk categories from the baseline data for assignment to R-HCVAD/MTX-ARA-C as consolidation in part 2 (4 cycles, 2 cycles, or no chemotherapy for high, medium and low risk pts respectively). Briefly, low risk pts were those with Ki-67 ≤30%, largest tumor mass <3 cm, low MIPI score and no features of high risk disease (Ki-67 ≥50%, mutations in the TP53, NSD2 or in NOTCH genes, complex karyotype or del17p, MYC positive, or largest tumor diameter >5 cm or blastoid/pleomorphic histology or if they remain in PR after 12 cycles of part 1. Medium risk are pts which did not belong to low or high-risk category. Those who experienced progression on part 1 went to part 2 and get 4 cycles of part 2. Patient were taken off protocol but not off study, if they remained in PR after 4 cycles of chemotherapy, these patients were followed up for time to next treatment and progression free survival on subsequent therapies. After part 2 consolidation, all pts received 2 years of IRV maintenance. The primary objective was to assess CR rates after IRV induction. Adverse events were coded as per CTCAE version 4. Molecular studies are being performed.
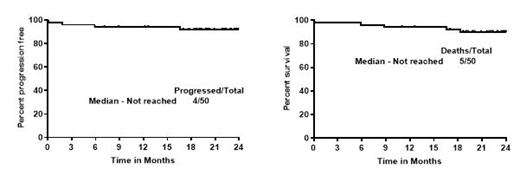
Results - Among the 50 pts, the median age was 57 years (range - 35-65). There were 20 pts in high-risk group, 20 pts in intermediate-risk group and 10 pts in low-risk group. High Ki-67 (≥30%) in 18/50 (36%) pts. Eighteen (36%) had high and intermediate risk simplified MIPI scores. Six (12%) pts had aggressive MCL (blastoid/pleomorphic). Among the 24 TP53 evaluable pts, eight pts (33%) had TP53 aberrations (mutated and/or TP53 deletion by FISH). Forty-eight pts received IRV. Best response to IRV was 96% and CR of 92%. After part 2, the best ORR remained unaltered, 96% (92% CR and 4% PR). The median number of cycles of triplet IRV to reach best response was 8 cycles (range 2-12). Fifteen pts (30%) did not receive part 2 chemotherapy, two pts (4%) received 1 cycle, 16 pts (32%) 2 cycles and 13 pts (26%) got 4 cycles of chemotherapy.
With a median follow up of 24 months, the median PFS and OS were not reached (2 year 92% and 90% respectively). The median PFS and OS was not reached and not significantly different in pts with high and low Ki-67% or with/without TP53 aberrations or among pts with low, medium or high-risk categories. The median PFS and OS was inferior in blastoid/pleomorphic MCL pts compared to classic MCL pts (p=0.01 and 0.03 respectively). Thirteen pts (26%) came off study - 5 for adverse events, 3 for on study deaths, and 2 for patient choice, 2 patients lost to follow up and one for disease progression. Overall, 5 pts died (3 on trial and 2 pts died off study, one due to progressive disease and another due to COVID pneumonia).
Grade 3-4 toxicities on part 1 were 10% myelosuppression and 10% each with fatigue, myalgia and rashes and 3% mucositis. One pt developed grade 3 atrial flutter on part 1. None had grade 3-4 bleeding/bruising.
Conclusions - Chemotherapy-free induction with IRV induced durable and deep responses in young MCL pts in the frontline setting. WINDOW-2 approach suggests that pts with low risk MCL do not need chemotherapy but further follow up is warranted. This combined modality treatment approach significantly improves outcomes of young MCL pts across all risk groups. Detailed molecular analyses will be reported.
Wang: Miltenyi Biomedicine GmbH: Consultancy, Honoraria; Bayer Healthcare: Consultancy; CStone: Consultancy; Celgene: Research Funding; Molecular Templates: Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Dava Oncology: Honoraria; Hebei Cancer Prevention Federation: Honoraria; Imedex: Honoraria; AstraZeneca: Consultancy, Honoraria, Research Funding; Epizyme: Consultancy, Honoraria; VelosBio: Consultancy, Research Funding; Mumbai Hematology Group: Honoraria; BGICS: Honoraria; Oncternal: Consultancy, Research Funding; Kite Pharma: Consultancy, Honoraria, Research Funding; DTRM Biopharma (Cayman) Limited: Consultancy; The First Afflicted Hospital of Zhejiang University: Honoraria; Lilly: Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; Anticancer Association: Honoraria; Juno: Consultancy, Research Funding; BioInvent: Research Funding; Loxo Oncology: Consultancy, Research Funding; Scripps: Honoraria; Physicians Education Resources (PER): Honoraria; OMI: Honoraria; Pharmacyclics: Consultancy, Research Funding; Newbridge Pharmaceuticals: Honoraria; Acerta Pharma: Consultancy, Honoraria, Research Funding; CAHON: Honoraria; Chinese Medical Association: Honoraria; Clinical Care Options: Honoraria; Moffit Cancer Center: Honoraria; Genentech: Consultancy; InnoCare: Consultancy, Research Funding. Jain: Lilly: Consultancy; kite: Consultancy. Iyer: CRISPRX: Research Funding; Seattle Genetics: Research Funding; Rhizen: Research Funding; Merck: Research Funding; Legend: Research Funding; Innate: Research Funding; Spectrum: Research Funding; Trillium: Research Funding; Astra Zeneca: Research Funding; Yingli: Research Funding; Cyclacel: Research Funding. Samaniego: Imbrium: Membership on an entity's Board of Directors or advisory committees; Arog: Research Funding. Vega: CRISPR Therapeutics and Geron: Research Funding; i3Health, Elsevier, America Registry of Pathology, Congressionally Directed Medical Research Program, and the Society of Hematology Oncology: Research Funding. Flowers: Gilead: Consultancy, Research Funding; Cancer Prevention and Research Institute of Texas: CPRIT Scholar in Cancer Research: Research Funding; Cellectis: Research Funding; Bayer: Consultancy, Research Funding; Eastern Cooperative Oncology Group: Research Funding; Novartis: Research Funding; Genmab: Consultancy; Pfizer: Research Funding; Xencor: Research Funding; TG Therapeutics: Research Funding; 4D: Research Funding; BeiGene: Consultancy; Genentech/Roche: Consultancy, Research Funding; Takeda: Research Funding; Celgene: Consultancy, Research Funding; National Cancer Institute: Research Funding; Spectrum: Consultancy; SeaGen: Consultancy; Karyopharm: Consultancy; Pharmacyclics/Janssen: Consultancy; Ziopharm: Research Funding; Burroughs Wellcome Fund: Research Funding; Epizyme, Inc.: Consultancy; Biopharma: Consultancy; Denovo: Consultancy; AbbVie: Consultancy, Research Funding; Acerta: Research Funding; Adaptimmune: Research Funding; Allogene: Research Funding; Sanofi: Research Funding; Amgen: Research Funding; Nektar: Research Funding; Morphosys: Research Funding; EMD: Research Funding; Kite: Research Funding; Guardant: Research Funding; Iovance: Research Funding; Janssen: Research Funding; Pharmacyclics: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal